by Jim Lane
Wandering the halls at the BIO World Congress and later to be seen again at ABLC NEXT this November, we ran across one of the most interesting technologies relating to ethanol production and markets we have seen in a month of Sundays, perhaps two months’ worth.

The problem
First, let’s revisit the problem. There’s simply too much ethanol being produced for the markets to absorb, given the Trump Administration’s massive cutbacks in US ethanol targets —In the resulting massively oversupplied market, the inevitable has happened, ethanol producers, growers and the Midwestern economies are being crushed. And they thought they felt left behind in 2016. Yikes.
The solution
Absent a shift of direction from Planet Trump, the solution is to crush the ethanol supply. There’s the painful route — which is shutting down plants, laying off workers, and booking John Mellencamp and friends for another series of Farm Aid concerts for the beleaguered growers.
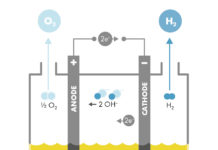
There’s the technically tougher path, which is to split ethanol and make CO2 and hydrogen. The point source, high-grade CO2 has value. The bio-based hydrogen has even more value than the ethanol.
Conceptually, the recipe is simple. Take ethanol, add water, apply energy, and split the resulting molecular broth into Hydrogen and CO2.
For armchair chemists, it looks like this:
C2H5OH + 3(H2O) —> 6H2 + 2(CO2)
Why is hydrogen valuable?
Well, think of it this way. The hydrogen captured in this process has more energy value than ethanol, and can power a hydrogen fuel cell vehicle that uses electric drive and is twice as energy efficient as an internal combustion engine.
We’ll mention parenthetically that the carbon intensity could go so low that you could be adding $3-$4 per gallon (well, gasoline gallon-equivalent) in low carbon credits in, say, the California market. Maybe more.
But let’s look beyond carbon value to energy value and mileage value.
First, at the Physics Factbook notes here, “Hydrogen has one of the highest energy density values per mass. Hydrogen has more energy per unit mass than other fuels (61,100 BTUs per pound versus 20,900 BTUs per pound of gasoline). The problem with hydrogen is that it is much less dense (pounds per gallon) than other fuels.
Second, as Edmunds.com observes, “Typically, a fuel-cell system is twice as efficient as a gasoline system. Most of the fuel-cell vehicles coming to market in the next few years will be able to deliver close to 70 miles per kilogram of fuel. That’s the equivalent of 70 miles per gallon.”
Put those together, and you have 103,000 theoretical BTUs in that hydrogen vs 76,000 BTUs in that original gallon of ethanol. Remember, we dragged more hydrogen out of the water in this catalytic reaction. If we get 85 percent efficiency in the reaction, we could capture around 87,000 BTUs. And, we double the efficiency because we are using the super-efficient fuel cell system.
So, we have 2.6 times as much energy value in that hydrogen, the way we just worked through the math. Plus, if you take gallons of the ethanol market without changing the EPA’s ethanol demand targets, and that ultra-clean CO2.
What’s the secret hidden value in this?
Consider that anhydrous ethanol can be supplied and then mixed with water, or a very hydrous ethanol could be supplied. More water, that’s less drying and a lower carbon intensity for the resulting hydrogen. If the ethanol comes from a particularly low-carbon feedstock, waste-based something — think carbon negative fuel.
The answer to the battery electric vehicle?
High-value, carbon-negative hydrogen fuel — yep, that’s the answer to the question, “why wouldn’t you drive a zero-emission vehicle?” Answer: because it doesn’t go far enough, fast enough on carbon.
Like ZEVs? (zero-emission vehicles). What about the negative emission vehicle, the NEV? Want to get rid of those CO2 megatonnes? Think Negatonnes.
Is the technology arriving?
Holy Hydrogen, Batman. Looks like we have a candidate, from the gang behind the SBI technology that Shell recently invested in. It’s a separate invention — but same people.
The Two Amazings. First, the catalyst can make the split, we’re told, and make it sufficiently fast and at a low enough temperature that there’s promise for bringing this to scale. Second, it uses highly hydrous, brothy ethanol, and uses the hydrogen in water to get the chemistry right.
The armchair chemist, of course, has already pointed out the alternative:
C2H5OH —> H2O + C2H4
Which is to say, dehydrate ethanol to make ethylene, which is one monumentally important molecule in the chemical industry. Now, you may have not frequently reflected on the opportunities for changing wine back into water — with a leftover of Glad Wrap (polyethylene), The problem is that you get four pounds of ethylene out of a gallon of ethanol, theoretically, the economics suck, and you lose the Renewable Fuel Standard credits.
What are the caveats and the emptors?
There are a bunch of “wait a minutes” on this one.
One, this process is on the bench, a long way from scale. Two, we don’t know as much about catalyst lifecycle as we will later. We don’t in this example have compressed hydrogen that is needed for vehicles. And, we don’t have a lot of fuel cell vehicles, distribution points, a commercially-scaled plant, access to pipelines, or an approved pathway under the Renewable Fuel Standard that has a whole bunch of difficulty handling renewable fuels made from bio-intermediates. Just to name a couple of hurdles.
And a friend adds:
It takes heat energy to drive the catalysis which means an endothermic reaction. The recent articles talking about a low temp catalysis is a huge step but even to get to 400 degrees F, a small amount of energy from the fuel cell is required to heat the catalysis. The fuel cell itself will create some heat energy but as I understand it, not at a high enough temp.
You can find out more.
They’re in Alberta and on a lab bench there. The guys down at International Stealth Mode HQ have this one under tight wraps, they might have to hold you at Gitmo after disclosing the details, but you can find out s’more at www.sbibioenergy.com.
Jim Lane is editor and publisher of Biofuels Digest where this article was originally published. Biofuels Digest is the most widely read Biofuels daily read by 14,000+ organizations. Subscribe here.